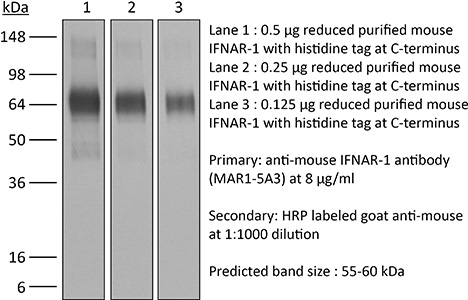
InVivoPlus anti-mouse IFNAR-1
| Clone | MAR1-5A3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalog # | BP0241 | ||||||||||
| Category | InVivoPlus Antibodies | ||||||||||
| Price |
|
The MAR1-5A3 monoclonal antibody reacts with mouse IFNAR-1 (IFN alpha/beta receptor subunit 1). IFNAR-1 is coexpressed with IFNAR-2 on nearly all cell types and together these two subunits make up the heterodimeric Type I IFN Receptor complex. Type I IFNs (IFN-α/β) bind to the Type I IFN Receptor complex to induce cellular responses including induction of anti-viral, anti-microbial, anti-tumor, and autoimmune responses as well as to regulate the activation, proliferation, and differentiation of many cell types. The MAR1-5A3 antibody has been shown to inhibit Type I IFN receptor signaling in vitro and in vivo.
| Isotype | Mouse IgG1, κ |
| Recommended Isotype Control(s) | InVivoPlus™ mouse IgG1 isotype control, unknown specificity |
| Recommended Dilution Buffer | InVivoPure™ pH 7.0 Dilution Buffer |
| Immunogen | Extracellular domain of mouse IFNAR-1 |
| Reported Applications |
|
| Formulation |
|
| Endotoxin |
|
| Aggregation |
|
| Purity |
|
| Sterility | 0.2 μM filtered |
| Production | Purified from tissue culture supernatant in an animal free facility |
| Purification | Protein G |
| RRID | AB_2687723 |
| Molecular Weight | 150 kDa |
| Murine Pathogen Test Results |
|
| Storage | The antibody solution should be stored at the stock concentration at 4°C. Do not freeze. |
INVIVOPLUS ANTI-MOUSE IFNAR-1 (CLONE: MAR1-5A3)
Macal, M., et al. (2018). “Self-Renewal and Toll-like Receptor Signaling Sustain Exhausted Plasmacytoid Dendritic Cells during Chronic Viral Infection.” Immunity 48(4): 730-744 e735. PubMed
Although characterization of T cell exhaustion has unlocked powerful immunotherapies, the mechanisms sustaining adaptations of short-lived innate cells to chronic inflammatory settings remain unknown. During murine chronic viral infection, we found that concerted events in bone marrow and spleen mediated by type I interferon (IFN-I) and Toll-like receptor 7 (TLR7) maintained a pool of functionally exhausted plasmacytoid dendritic cells (pDCs). In the bone marrow, IFN-I compromised the number and the developmental capacity of pDC progenitors, which generated dysfunctional pDCs. Concurrently, exhausted pDCs in the periphery were maintained by self-renewal via IFN-I- and TLR7-induced proliferation of CD4(-) subsets. On the other hand, pDC functional loss was mediated by TLR7, leading to compromised IFN-I production and resistance to secondary infection. These findings unveil the mechanisms sustaining a self-perpetuating pool of functionally exhausted pDCs and provide a framework for deciphering long-term exhaustion of other short-lived innate cells during chronic inflammation.
Liu, X., et al. (2015). “CD47 blockade triggers T cell-mediated destruction of immunogenic tumors.” Nat Med 21(10): 1209-1215. PubMed
Macrophage phagocytosis of tumor cells mediated by CD47-specific blocking antibodies has been proposed to be the major effector mechanism in xenograft models. Here, using syngeneic immunocompetent mouse tumor models, we reveal that the therapeutic effects of CD47 blockade depend on dendritic cell but not macrophage cross-priming of T cell responses. The therapeutic effects of anti-CD47 antibody therapy were abrogated in T cell-deficient mice. In addition, the antitumor effects of CD47 blockade required expression of the cytosolic DNA sensor STING, but neither MyD88 nor TRIF, in CD11c(+) cells, suggesting that cytosolic sensing of DNA from tumor cells is enhanced by anti-CD47 treatment, further bridging the innate and adaptive responses. Notably, the timing of administration of standard chemotherapy markedly impacted the induction of antitumor T cell responses by CD47 blockade. Together, our findings indicate that CD47 blockade drives T cell-mediated elimination of immunogenic tumors.
Schliehe, C., et al. (2015). “The methyltransferase Setdb2 mediates virus-induced susceptibility to bacterial superinfection.” Nat Immunol 16(1): 67-74. PubMed
Immune responses are tightly regulated to ensure efficient pathogen clearance while avoiding tissue damage. Here we report that Setdb2 was the only protein lysine methyltransferase induced during infection with influenza virus. Setdb2 expression depended on signaling via type I interferons, and Setdb2 repressed expression of the gene encoding the neutrophil attractant CXCL1 and other genes that are targets of the transcription factor NF-kappaB. This coincided with occupancy by Setdb2 at the Cxcl1 promoter, which in the absence of Setdb2 displayed diminished trimethylation of histone H3 Lys9 (H3K9me3). Mice with a hypomorphic gene-trap construct of Setdb2 exhibited increased infiltration of neutrophils during sterile lung inflammation and were less sensitive to bacterial superinfection after infection with influenza virus. This suggested that a Setdb2-mediated regulatory crosstalk between the type I interferons and NF-kappaB pathways represents an important mechanism for virus-induced susceptibility to bacterial superinfection.
Welten, S. P., et al. (2015). “The viral context instructs the redundancy of costimulatory pathways in driving CD8(+) T cell expansion.” Elife 4. doi: 10.7554/eLife.07486. PubMed
Signals delivered by costimulatory molecules are implicated in driving T cell expansion. The requirements for these signals, however, vary from dispensable to essential in different infections. We examined the underlying mechanisms of this differential T cell costimulation dependence and found that the viral context determined the dependence on CD28/B7-mediated costimulation for expansion of naive and memory CD8(+) T cells, indicating that the requirement for costimulatory signals is not imprinted. Notably, related to the high-level costimulatory molecule expression induced by lymphocytic choriomeningitis virus (LCMV), CD28/B7-mediated costimulation was dispensable for accumulation of LCMV-specific CD8(+) T cells because of redundancy with the costimulatory pathways induced by TNF receptor family members (i.e., CD27, OX40, and 4-1BB). Type I IFN signaling in viral-specific CD8(+) T cells is slightly redundant with costimulatory signals. These results highlight that pathogen-specific conditions differentially and uniquely dictate the utilization of costimulatory pathways allowing shaping of effector and memory antigen-specific CD8(+) T cell responses.
Yang, H., et al. (2015). “STAT3 Inhibition Enhances the Therapeutic Efficacy of Immunogenic Chemotherapy by Stimulating Type 1 Interferon Production by Cancer Cells.” Cancer Res 75(18): 3812-3822. PubMed
STAT3 is an oncogenic transcription factor with potent immunosuppressive functions. We found that pharmacologic inhibition of STAT3 or its selective knockout in cancer cells improved the tumor growth-inhibitory efficacy of anthracycline-based chemotherapies. This combined effect of STAT3 inhibition/depletion and anthracyclines was only found in tumors growing on immunocompetent (not in immunodeficient) mice. As compared with Stat3-sufficient control tumors, Stat3(-/-) cancer cells exhibited an increased infiltration by dendritic cells and cytotoxic T lymphocytes after chemotherapy. Anthracyclines are known to induce several stress pathways that enhance the immunogenicity of dying and dead cancer cells, thereby stimulating a dendritic cell-dependent and T lymphocyte-mediated anticancer immune response. Among these therapy-relevant stress pathways, Stat3(-/-) cancer cells manifested one significant improvement, namely an increase in the expression of multiple type-1 interferon-responsive genes, including that of the chemokines Cxcl9 and Cxcl10. This enhanced type-1 interferon response could be suppressed by reintroducing wild-type Stat3 (but not a transactivation-deficient mutant Stat3(Y705F)) into the tumor cells. This maneuver also abolished the improved chemotherapeutic response of Stat3(-/-) cancers. Finally, the neutralization of the common type-1 interferon receptor or that of the chemokine receptor CXCR3 (which binds CXCL9 and CXCL10) abolished the difference in the chemotherapeutic response between Stat3(-/-) and control tumors. Altogether, these results suggest that STAT3 inhibitors may improve the outcome of chemotherapy by enhancing the type-1 interferon response of cancer cells.
Beug, S. T., et al. (2014). “Smac mimetics and innate immune stimuli synergize to promote tumor death.” Nat Biotechnol 32(2): 182-190. PubMed
Smac mimetic compounds (SMC), a class of drugs that sensitize cells to apoptosis by counteracting the activity of inhibitor of apoptosis (IAP) proteins, have proven safe in phase 1 clinical trials in cancer patients. However, because SMCs act by enabling transduction of pro-apoptotic signals, SMC monotherapy may be efficacious only in the subset of patients whose tumors produce large quantities of death-inducing proteins such as inflammatory cytokines. Therefore, we reasoned that SMCs would synergize with agents that stimulate a potent yet safe “cytokine storm.” Here we show that oncolytic viruses and adjuvants such as poly(I:C) and CpG induce bystander death of cancer cells treated with SMCs that is mediated by interferon beta (IFN-beta), tumor necrosis factor alpha (TNF-alpha) and/or TNF-related apoptosis-inducing ligand (TRAIL). This combinatorial treatment resulted in tumor regression and extended survival in two mouse models of cancer. As these and other adjuvants have been proven safe in clinical trials, it may be worthwhile to explore their clinical efficacy in combination with SMCs.
Calame, D. G., et al. (2014). “The C5a anaphylatoxin receptor (C5aR1) protects against Listeria monocytogenes infection by inhibiting type 1 IFN expression.” J Immunol 193(10): 5099-5107. PubMed
Listeria monocytogenes is a major cause of mortality resulting from food poisoning in the United States. In mice, C5 has been genetically linked to host resistance to listeriosis. Despite this genetic association, it remains poorly understood how C5 and its activation products, C5a and C5b, confer host protection to this Gram-positive intracellular bacterium. In this article, we show in a systemic infection model that the major receptor for C5a, C5aR1, is required for a normal robust host immune response against L. monocytogenes. In comparison with wild-type mice, C5aR1(-/-) mice had reduced survival and increased bacterial burden in their livers and spleens. Infected C5aR1(-/-) mice exhibited a dramatic reduction in all major subsets of splenocytes, which was associated with elevated caspase-3 activity and increased TUNEL staining. Because type 1 IFN has been reported to impede the host response to L. monocytogenes through the promotion of splenocyte death, we examined the effect of C5aR1 on type 1 IFN expression in vivo. Indeed, serum levels of IFN-alpha and IFN-beta were significantly elevated in L. monocytogenes-infected C5aR1(-/-) mice. Similarly, the expression of TRAIL, a type 1 IFN target gene and a proapoptotic factor, was elevated in NK cells isolated from infected C5aR1(-/-) mice. Treatment of C5aR1(-/-) mice with a type 1 IFNR blocking Ab resulted in near-complete rescue of L. monocytogenes-induced mortality. Thus, these findings reveal a critical role for C5aR1 in host defense against L. monocytogenes through the suppression of type 1 IFN expression.
Ma, Y., et al. (2014). “Borrelia burgdorferi arthritis-associated locus Bbaa1 regulates Lyme arthritis and K/BxN serum transfer arthritis through intrinsic control of type I IFN production.” J Immunol 193(12): 6050-6060. PubMed
Localized upregulation of type I IFN was previously implicated in development of Borrelia burgdorferi-induced arthritis in C3H mice, and was remarkable due to its absence in the mildly arthritic C57BL/6 (B6) mice. Independently, forward genetics analysis identified a quantitative trait locus on Chr4, termed B. burgdorferi-associated locus 1 (Bbaa1), that regulates Lyme arthritis severity and includes the 15 type I IFN genes. Involvement of Bbaa1 in arthritis development was confirmed in B6 mice congenic for the C3H allele of Bbaa1 (B6.C3-Bbaa1), which developed more severe Lyme arthritis and K/BxN model of rheumatoid arthritis (RA) than did parental B6 mice. Administration of a type I IFN receptor blocking mAb reduced the severity of both Lyme arthritis and RA in B6.C3-Bbaa1 mice, formally linking genetic elements within Bbaa1 to pathological production of type I IFN. Bone marrow-derived macrophages from Bbaa1 congenic mice implicated this locus as a regulator of type I IFN induction and downstream target gene expression. Bbaa1-mediated regulation of IFN-inducible genes was upstream of IFN receptor-dependent amplification; however, the overall magnitude of the response was dependent on autocrine/paracrine responses to IFN-beta. In addition, the Bbaa1 locus modulated the functional phenotype ascribed to bone marrow-derived macrophages: the B6 allele promoted expression of M2 markers, whereas the C3H allele promoted induction of M1 responses. This report identifies a genetic locus physically and functionally linked to type I IFN that contributes to the pathogenesis of both Lyme and RA.
Stock, A. T., et al. (2014). “Type I IFN suppresses Cxcr2 driven neutrophil recruitment into the sensory ganglia during viral infection.” J Exp Med 211(5): 751-759. PubMed
Infection induces the expression of inflammatory chemokines that recruit immune cells to the site of inflammation. Whereas tissues such as the intestine and skin express unique chemokines during homeostasis, whether different tissues express distinct chemokine profiles during inflammation remains unclear. With this in mind, we performed a comprehensive screen of the chemokines expressed by two tissues (skin and sensory ganglia) infected with a common viral pathogen (herpes simplex virus type 1). After infection, the skin and ganglia showed marked differences in their expression of the family of Cxcr2 chemokine ligands. Specifically, Cxcl1/2/3, which in turn controlled neutrophil recruitment, was up-regulated in the skin but absent from the ganglia. Within the ganglia, Cxcl2 expression and subsequent neutrophil recruitment was inhibited by type I interferon (IFN). Using a combination of bone marrow chimeras and intracellular chemokine staining, we show that type I IFN acted by directly suppressing Cxcl2 expression by monocytes, abrogating their ability to recruit neutrophils to the ganglia. Overall, our findings describe a novel role for IFN in the direct, and selective, inhibition of Cxcr2 chemokine ligands, which results in the inhibition of neutrophil recruitment to neuronal tissue.